Animals and Water
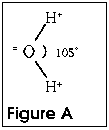 Water is one of the more unusual materials in the creation. Because
the molecule is V-shaped, as shown in Figure A, it is polar--has
a positively charged end and a negatively charged end. If you
bring a charged rod near a stream of water flowing through the
air, the water will change direction due to the charge. A negatively
charged rod will repel the negatively charged oxygen atom and
the water molecule is pushed away.
Water is one of the more unusual materials in the creation. Because
the molecule is V-shaped, as shown in Figure A, it is polar--has
a positively charged end and a negatively charged end. If you
bring a charged rod near a stream of water flowing through the
air, the water will change direction due to the charge. A negatively
charged rod will repel the negatively charged oxygen atom and
the water molecule is pushed away.
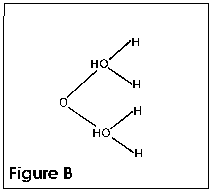 The polar nature of the water molecule allows all kinds of interesting
properties of water itself. When water freezes, for example,
the molecules are attracted to each other as shown in Figure B.
To allow this attraction to take place, the volume has to increase.
This causes water to expand when it freezes. When a solid like
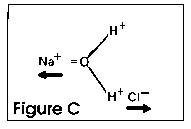
salt is put into water, it is ripped apart by the polar molecule
as shown in Figure C. The positive sodium is pulled to the oxygen
side of the water molecule, and the negative chloride is pulled
to the hydrogen side. This rips apart the salt mole-cule in a
process we call dissolving, which is critical to the survival
of life on planet Earth.
The polar nature of the water molecule allows all kinds of interesting
properties of water itself. When water freezes, for example,
the molecules are attracted to each other as shown in Figure B.
To allow this attraction to take place, the volume has to increase.
This causes water to expand when it freezes. When a solid like
salt is put into water, it is ripped apart by the polar molecule
as shown in Figure C. The positive sodium is pulled to the oxygen
side of the water molecule, and the negative chloride is pulled
to the hydrogen side. This rips apart the salt mole-cule in a
process we call dissolving, which is critical to the survival
of life on planet Earth.
Living things are designed to take advantage of the properties
of the water molecule. In the Anarctic coastal waters are fish
which live in water at 28ûF. These fish have in their blood
stream molecules of antifreeze which have glycoproteins which
are 300 times as effective as the antifreeze in your car radiator.
These fish, called notothenioids, produce the glycoprotein in
their livers. It is secreted into their bloodstream and fills
the spaces around cells. Water in the fish's blood freezes, but
the glycoprotein stops the ice crystals from growing and they
are eventually filtered out.1 This non-polar molecule prevents
the polar water molecules from attaching to each other and allows
abundant life in an environment which would seem to stop all life
from existing.
Desert creatures also take advantage of the unique properties
of water to allow survival in very hot dry places. The horned
toad of Texas (which is actually a lizard) is an example. Because
of water's polar nature, it is attracted to the sides of its container,
a process called adhesion. If the container is small enough that
all sides pull on the water molecules, the molecules can actually
move up the container, a process called capillary action.
When it rains, the horned toad will spread its legs out to the
sides, lower its head, and arch its back. When the rain is over,
the toad will drop down to the ground and rub its belly in the
moist sand. Both of the actions get the toad water. Thin hair-like
channels between the toad's scales run to its mouth. Any water
that touches any part of the toad's anatomy is drawn to its mouth
by capillary action. With any moisture at all being present,
all the toad has to do is stand or lie still and swallow. The
design of its body causes water to flow to its mouth.2
There are countless other designs for obtaining water, but it
is the design of the water molecule itself that makes most of
them possible. The complexity of the systems that we see at an
atomic and molecular level and also on the level of the animals
and plants themselves testifies to the impossibility of chance
explaining the creation. If chance is an invalid mechanism, then
design is the only alternative. The design we see demands a designer--a
personal God who had a purpose in all He did and the intelligence
to do it.
1National Geographic, June, 1995, page 3.
2The Quest for Water" by Doug Stewart, National Wildlife,
June/July, 1995, pages 30-33.
Back to Contents Does God Exist?, Jul/Aug 1996
 Water is one of the more unusual materials in the creation. Because
the molecule is V-shaped, as shown in Figure A, it is polar--has
a positively charged end and a negatively charged end. If you
bring a charged rod near a stream of water flowing through the
air, the water will change direction due to the charge. A negatively
charged rod will repel the negatively charged oxygen atom and
the water molecule is pushed away.
Water is one of the more unusual materials in the creation. Because
the molecule is V-shaped, as shown in Figure A, it is polar--has
a positively charged end and a negatively charged end. If you
bring a charged rod near a stream of water flowing through the
air, the water will change direction due to the charge. A negatively
charged rod will repel the negatively charged oxygen atom and
the water molecule is pushed away.
 The polar nature of the water molecule allows all kinds of interesting
properties of water itself. When water freezes, for example,
the molecules are attracted to each other as shown in Figure B.
To allow this attraction to take place, the volume has to increase.
This causes water to expand when it freezes. When a solid like
salt is put into water, it is ripped apart by the polar molecule
as shown in Figure C. The positive sodium is pulled to the oxygen
side of the water molecule, and the negative chloride is pulled
to the hydrogen side. This rips apart the salt mole-cule in a
process we call dissolving, which is critical to the survival
of life on planet Earth.
The polar nature of the water molecule allows all kinds of interesting
properties of water itself. When water freezes, for example,
the molecules are attracted to each other as shown in Figure B.
To allow this attraction to take place, the volume has to increase.
This causes water to expand when it freezes. When a solid like
salt is put into water, it is ripped apart by the polar molecule
as shown in Figure C. The positive sodium is pulled to the oxygen
side of the water molecule, and the negative chloride is pulled
to the hydrogen side. This rips apart the salt mole-cule in a
process we call dissolving, which is critical to the survival
of life on planet Earth.
