
One of the more interesting areas of physics is the study of the forces
that are built into the creation. Famous scientists like Einstein, Millikan,
Coulomb, Maxwell, Michelson, Morely, Bohr, Newton, and many others have
all contributed to our understanding of what causes the forces and how
they relate to one another. Gravity, electricity, and magnetism are some
of the subjects which these famous people have studied and explained so
that mankind can control his environment and provide security and food
for himself.

A good starting point for what we know about all these forces is the atom, and most of us were introduced to what we know about science by describing the atom's properties. The atom is mostly space. Figure 1 might give you some idea of what this means. The dot represents the nucleus of the atom, and the circle represents the orbit of the first electron sometimes called the S orbital electron. Two electrons can fit into this orbital moving in a roughly circular orbit. If we moved further out than this orbital, we would find a lot more space and then we would find another energy level that would once again have the ability to hold two electrons moving in a circular orbit.
If you were to travel still further out, you would find still more space.
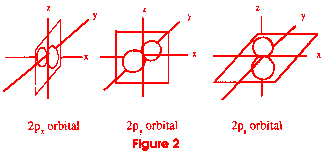
You would eventually come to another energy level of electrons. This level
would hold up to six electrons, but the electrons would travel in three
figure 8 patterns, all at right angles to each other (see figure 2). An
atom that had all of these electron levels full would have ten electrons--two
in the first s orbital, two in the second, and six in the p orbital. These
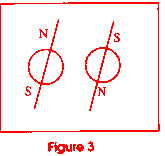
electrons have another interesting property, and that is that they are
magnetically paired. Electrons have a north and a south pole which is controlled
by the way they spin on their axes (see figure 3). There are three pairs
of electrons in the p orbital, and one pair in each of the other two levels.
When all of these electrons are paired and all the levels are full, the
atom is totally stable. The material is inert and does not involve itself
in a chemical reaction. This material is called Neon and it is an inert
gas.
Suppose we had a material that was missing one electron in the p orbital.
This material would need a single electron to fill that p shell, and it
would try very hard to get it. This desire to fill that shell makes the
substance chemically active, and the material will attack almost any other
atom to get the one electron it needs. If you had an atom like hydrogen
that had only one electron, the material missing an electron would share
one of its electrons with the hydrogen using the hydrogen electron to fill
its own missing electron.
The example we have used is the element fluorine combining with hydrogen to make hydrogen fluoride. If an atom were missing two p electrons, it would be the element oxygen, and it would need two hydrogens to fill its missing p level openings. This combination produces water. We have over-simplified the picture and have chosen the simplest cases to show how the system works. Some atoms have a shell called the d shell that can hold up to 10 electrons which orbit in a clover leaf pattern. There is still another level called the f level that can hold up to 14 electrons. These energy levels can overlap, and electrons can move from one level to another because of the overlaps.
I predict that you have made one of three responses to the previous
paragraphs. Those responses (I predict) were:
Well, no matter which of those three choices you are closest to, we
can all take home one fundamental point. This is an incredibly complex
system. The interesting theory is that it is this complexity that allows
mankind to exist and the earth to be lived on. Rocks, food, air, and our
bodies exist because of the many ways electrons can be arranged. Just trying
to understand how the system works is difficult. Imagine trying to design
it from scratch so that it will work.
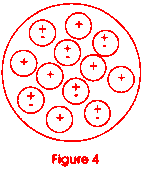
Now go back to figure 1 and look at it again. Let us look at the dot in the center of the drawing. If you could reduce yourself to the size of the dot and look inside it, you would see that it is made up of positive charged particles called protons and neutrally charged particles called neutrons. Figure 4 shows us what the nucleus of the carbon atom might look like. It has six protons and six neutrons. All of the protons have the same charge and like charges repel! This is an unstable situation with a potentially explosive nature. Figure 5 shows a famous equation derived by a man named Coulomb. It enables us to calculate how much force two charged particles (called q and q') would have if they were some distance (x) apart. In the nucleus of the atom, things are very close together (look at Figure 1; the dot is very small). The value of k is huge (109 is a billion). Why does the nucleus not just fly apart?
The answer is that there is a very powerful force unimaginably called
nuclear force which holds it together. The energy that produces this force
comes from Einstein's famous equation E = mc2. Mass is converted
into energy that provides the force which holds the nucleus together.
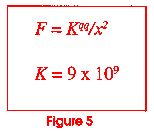
Now go back to the three responses that I predicted would be made a few paragraphs back. Those who made response 1 are likely to be saying "Oh, man, I remember how mind-boggling that stuff was." Those who made response 2 are likely to be saying "That's it, I quit." And those who made response 3 are likely to be saying "I think you are all out of your mind." Actually, this last explanation is very over-simplified, totally incomplete, and probably somewhat misleading. The point we can all take from this is that, complicated as the electron structure that allows chemistry, the nuclear structure that has led to nuclear power, medical cures, and smoke alarms is vastly more complicated--and, in fact, there is still much to learn.
Could an atom exist if it were made any other way? The answer is "No." In order for atoms to make molecules and molecules to allow the arrangements necessary to make the compounds necessary for matter to exist, the atom has to be designed just as it is. If the value of K in figure 5 were less than 9 x 109, the nuclear forces would crush the nucleus. If the constants for magnetic forces were any different than they are, electrons could not be paired to make molecules. There are over 40 different constants that describe the properties matter has to have for an atom to exist. (Note--for a table listing these constants, send a self-addressed 32¢ stamped envelope to John Clayton, 1555 Echo Valley Dr., Niles MI 49120), and we will send it to you.)
Several years ago, I had a chemistry teacher who lectured us for two days on the structure of the atom. At the end of his lecture, he said, "How do you make an atom? Ladies and gentlemen, it is not easy." But, with God, all things are possible.
Back to Contents Does God Exist?, Jan/Feb98.